 Featured News September 04, 2014
Med BioGene Announces Receipt of Proxies for Upcoming Annual and Special Meeting
August 06, 2014
Med BioGene Announces Director Nominations and Postponement of Annual and Special Meeting
|
GeneFx® Lung
GeneFx® Lung uses our proprietary 15-gene signature to improve upon staging for identifying those patients with early-stage non-small-cell lung cancer (NSCLC) who, following surgical removal of their tumor, are at a higher and lower risk of mortality. Early-stage NSCLC patients are treated primarily by surgical removal of their tumours. Recent clinical trials have established that adjuvant chemotherapy, administered after tumour removal, significantly improves the survival of stage II patients, but does not significantly improve the survival of stage I patients. As a result, the American Society of Clinical Oncology and National Comprehensive Cancer Network recommend adjuvant chemotherapy for stage II patients but not for stage I patients. However, 30% to 55% of stage I and II patients still die as a result of the disease, implying that patients diagnosed with the same stage of disease can have markedly different treatment responses and overall outcomes. Currently, tumor stage (determined by the size and location of the tumour and lymph node involvement) remains the strongest predictor of survival but fails to account for this difference in patient outcomes. GeneFx Lung is expected to help address this critical issue.
Of the 482 patients participating in the JBR.10 clinical trial, tumor specimens from 62 observation patients and 71 patients treated with chemotherapy were available for analysis. The GeneFx Lung genes were selected using an unbiased genome - wide approach in which all 25,000 genes in the human genome from the observation patients were evaluated and narrowed down to 172 genes with the greatest ability to predict mortality. Using statistical analysis, a sub-set of 15 genes were shown to best correlate with patient survival and were selected for the GeneFx Lung signature. Using the expression levels of these 15 genes, a proprietary algorithm was developed that separates patients into higher and lower risk groups based upon predicted mortality. GeneFx Lung was subsequently validated in predicting patient mortality in five independent studies involving data from tumor specimens totaling 676 untreated early-stage NSCLC patients. The 71 JBR.10 clinical trial patients treated with chemotherapy were also compared to the 62 observation patients using the GeneFx Lung classification (see graphs above). This study demonstrated that patients classified by GeneFx Lung as higher risk significantly benefited from adjuvant chemotherapy, and those classified as lower risk did not benefit, and may have experienced a detrimental effect, from adjuvant chemotherapy. MBI believes that the results of these studies demonstrate that GeneFx Lung will result in better-informed and personalized treatment decisions and improve the selection of patients who may benefit from chemotherapy. GeneFx Lung is being developed in collaboration with a team of internationally acclaimed researchers and physicians at the Princess Margaret Hospital in Toronto. The team is led by Dr. Ming-Sound Tsao, holder of the M. Qasim Choksi Chair in Lung Cancer Translational Research, and Dr. Frances A. Shepherd, holder of the Scott Taylor Chair in Lung Cancer Research and the Past-Chair of the National Cancer Institute of Canada Clinical Trials Group Lung Cancer Site. Drs. Tsao and Shepherd are Professors at the University of Toronto and have in total authored more than 500 articles in peer reviewed journals. Princess Margaret Hospital, a research hospital of the University of Toronto, has achieved an international reputation as a global leader in the fight against cancer and is considered one of the top comprehensive cancer treatment and research centres in the world. Located in Toronto, Princess Margaret Hospital, together with its research institute, the Ontario Cancer Institute, is a member of the University Health Network, which also includes the Toronto General Hospital and the Toronto Western Hospital. Princess Margaret Hospital is the only facility in Canada devoted exclusively to cancer research, treatment and education. |
|
© Copyright 2006 - Med BioGene Inc. - Legal Disclaimer





 Home
Home 
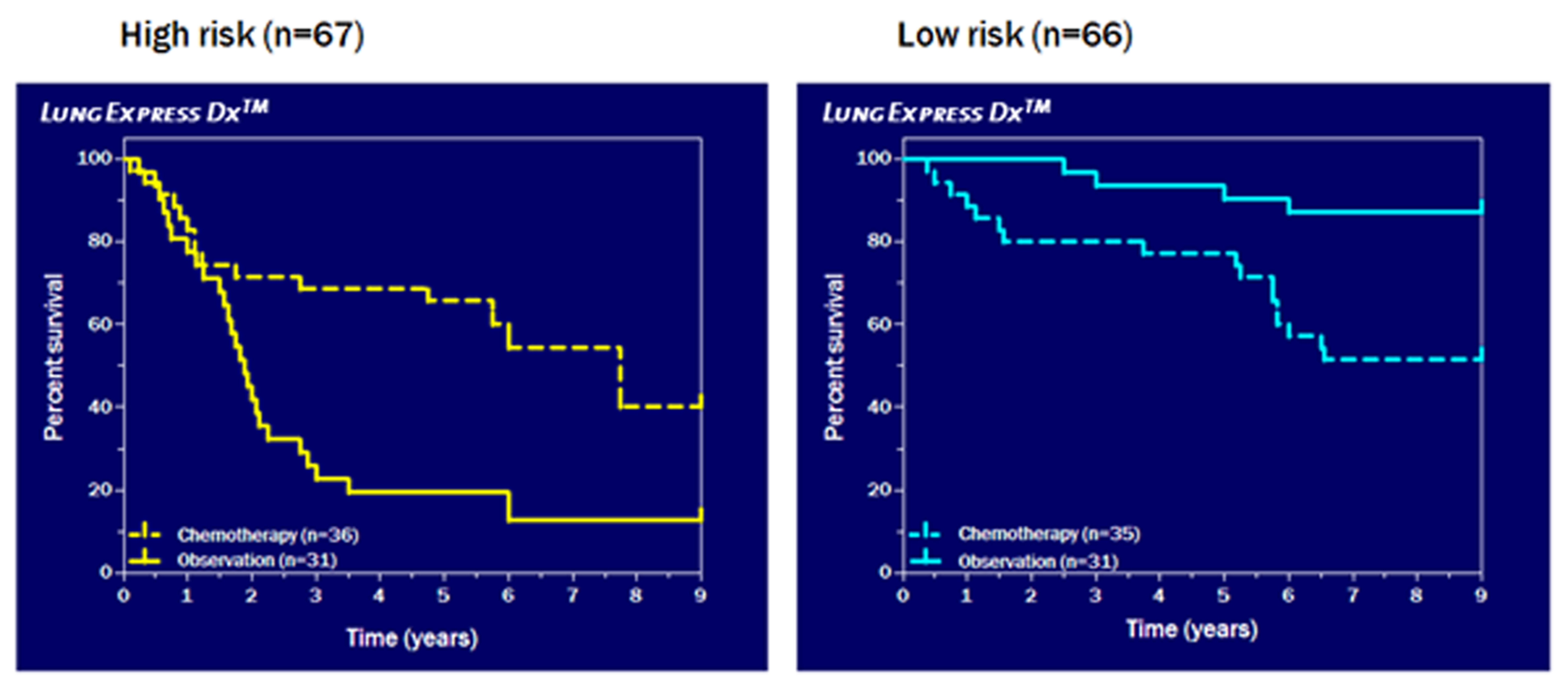 As published in the Journal of Clinical Oncology with an accompanying editorial, and presented elsewhere, the 15-gene signature of GeneFx Lung was developed using five-year outcome data from untreated early-stage NSCLC patients from the National Cancer Institute of Canada Clinical Trials Group JBR.10 trial.
As published in the Journal of Clinical Oncology with an accompanying editorial, and presented elsewhere, the 15-gene signature of GeneFx Lung was developed using five-year outcome data from untreated early-stage NSCLC patients from the National Cancer Institute of Canada Clinical Trials Group JBR.10 trial.
